A study from the Biotechnology Center (BIOTEC) of TUD Dresden University of Technology and the Institute of Cancer Research revealed that a subtle chemical change to protein p21 acts as a master regulator of cell division.
This discovery could have widespread implications for cancer therapies. The group found that this slight change determines whether cells continue to grow or enter senescence.
Redox proteomics was used to map how reactive oxygen species chemically modify proteins. Although these molecules are linked to cell damage, they can also act as important messengers that play a role in regulating cellular processes like stress response and cell division.
Over 1,700 individual oxidation sites were labelled and analysed across various proteins. This enables researchers to track their changes throughout the entire cell cycle. As a result, they created one of the most intricate maps of redox activity during cell division.
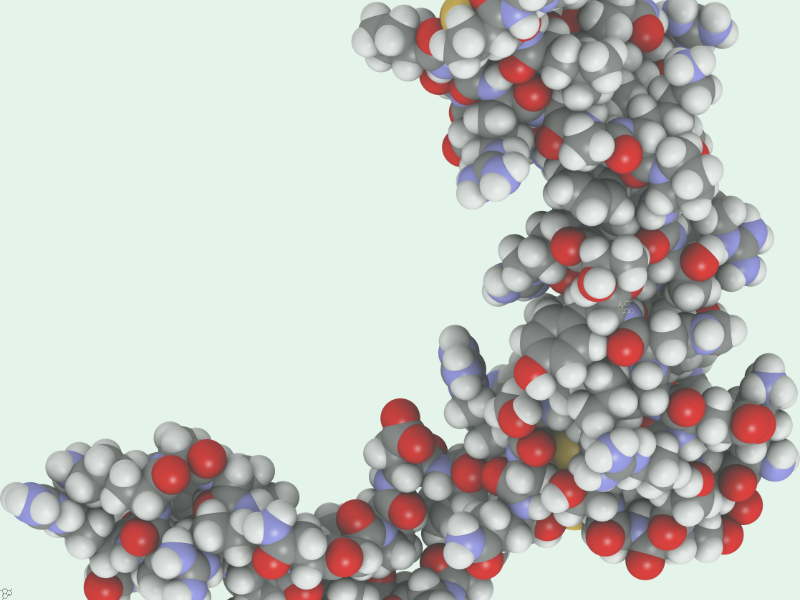
Upon focusing on p21, they noticed that its oxidation reached its peak just before the cells divided. The study found that the oxidation of a single site on p21, a cysteine amino acid at position 41 (C41), is key to influencing how the protein behaves and engages with other proteins.
When this site is oxidised, which occurs immediately before a cell divides, p21 breaks down, allowing cells to keep reproducing. However, when the site is not oxidised as a result of a mutation or lack of reactive oxygen, p21 becomes more stable and cells are more likely to enter senescence.
“By understanding how p21’s oxidation state controls its stability and interactions, we’ve uncovered a completely new layer of regulation in the cell cycle. This could be a useful target in cancer, especially in tumours where p21 is present but misregulated,” said Dr Julia Vorhauser, co-lead author and Postdoctoral Training Fellow in the Post-translational Modifications and Cell Proliferation Group.